 RDO Springs - 10 Rue Benjamin Delessert - 60510 BRESLES - FRANCE
RDO Springs - 10 Rue Benjamin Delessert - 60510 BRESLES - FRANCE
Our quality approach | RDO Springs French spring specialist
Quality System
The result of rigorous work concerning the requirements of the aerospace sector, RDO Springs is certified according to the ISO 9001 and EN 9100 international standards.
Depending on your requirements, we can provide a certificate of compliance, an inspection report and the corresponding raw material certificate on delivery.
The principles of continuous improvement (PDCA) are applied at all levels of the company.

Traceability
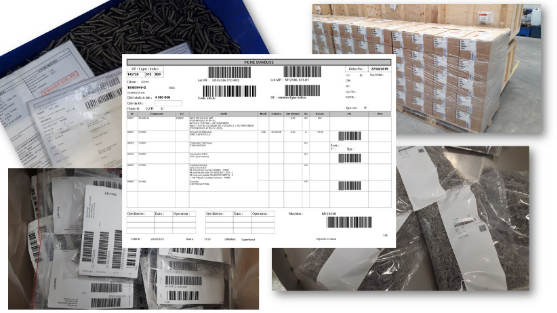
Our data is integrated in our ERP. Consequently parts are completely traced so we know exactly which components are in our products and all the data used in their manufacture and delivery.
Production and control ranges are created for each article ensuring instructions are transmitted correctly for the products’ adjustment, production and quality control operations based on customers’ requirements.
Our products are identified by barcodes on the boxes, but also on the bags in case of passivation operations.
Change management, analysis, validation
RDO Springs’ approach involves making its processes as reliable as possible at each stage of product production. This involves controlling changes made during products’ model life, including configuration management.
In the same way, we support our customers in the validation of new products: prototypes, initial inspections (F.A.I.), initial samples (E.I), etc.
RDO Springs has also committed to comply with “change control” procedures. An engineer in charge of validations systematically validates in the pharmaceutical sense of the term whenever a modification, even a minor one, of the manufacturing process is envisaged.
These procedures include:
- Risk analysis.
- Drafting a qualification protocol.
- Performing qualification tests.
Good Manufacturing Practices
RDO Springs has made a commitment to its pharmaceutical customers to respect G.M.P. (Good Manufacturing Practices ) that encompass all the control, quality, traceability and cleanliness requirements specific to this industry.
Each employee is encouraged to comply with Good Manufacturing Practices, in order to limit as much as possible the risks of deviation during production, in particular :
Limit the risks of contamination of the products by another product, or an internal and external contaminant (rules of hygiene and cleanliness).
Limit the risk of confusion, particularly with regard to labelling and component identification.
Adapt and maintain equipment and premises to avoid contamination.
Our Production Strengths

High level of control of our production processes

Precision measuring equipment

Good Manufacturing Practices
RDO Springs
10 RUE BENJAMIN DELESSERT
60510 BRESLES
contact@rdosprings.com
www.rdosprings.com
 (+33) 3 44 07 34 34
(+33) 3 44 07 34 34 contact@rdosprings.com
contact@rdosprings.com


 REQUEST FOR QUOTATION
REQUEST FOR QUOTATION download the brochure
download the brochure